our mission
Beginning Strong
Living Longer
At Vitaleon Pharma, we are dedicated to pioneering advancements in fertility and overall wellbeing. Our mission is to not only spark the miracle of life but to ensure its longevity and vitality. By improving fertility, ensuring healthy telomere extension in newborns and combating the challenges of NASH, we’re striving for a world where every new life starts stronger and every life journey is healthier.
benefits of bgp-15
Energize, Protect, Revitalize:
Empowering mitochondria, neutralizing cellular stress, calming Inflammation
About the drug
BGP-15 - A Promising Active Pharmaceutical Ingredient
About the drug
BGP-15 - A Promising Active Pharmaceutical Ingredient
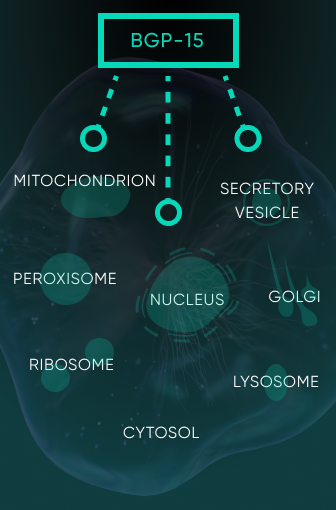
BGP-15 stands out as a potential game-changer in the pharmaceutical landscape due to its multifaceted therapeutic properties. At the cellular level, it
is known to facilitate mitochondrial fusion, reduce the production of reactive oxygen species, elevate reduced glutathione levels, increase mitochondrial
respiratory complex I activity and boost the expression of heat shock proteins.
Having undergone five human clinical trials, including two phase 2 studies, BGP-15 has been administered to over 200 patients and volunteers.
Impressively, it has maintained a favourable safety profile across these trials, even at high doses.
As of now, a phase 2 clinical study is on the horizon for 2024, aiming to explore BGP-15’s efficacy in enhancing male fertility and improving newborn
health.
Additionally, its potential in preventing NASH-induced hepatocellular carcinoma, and treating rare paediatric conditions like Familial Dysautonomia and Duchenne muscular dystrophy make it a compound of significant promise.
previous clinical trails
BGP-15 was tested for
safety and efficacy in 5 human clinical trials
Phase 1A Single Dose-Escalation Tolerability Study • 16 healthy male volunteers
• Single doses up to 800 mg were well tolerated.
Phase 1B Multiple Doses Tolerability Study • 16 healthy male volunteers
• 250, 600 mg doses daily for 7 days
Phase 1 Randomized Double Blind, Placebo-Controlled • 42 healthy individuals
• Olanzapine + placebo and olanzapine + BGP 15 400 mg daily for 17 days
Phase 2A Randomized Double-Blind, Placebo-Controlled Study
• 42 insulin resistant patients • 200 or 400 mg BGP-15 or placebo daily for 28 days
Phase 2B Randomized, Double blind, Placebo controlled • 196 diabetic patients
• 100, 200 or 400 mg BGP-15 or placebo daily for 13 weeks
Single Dose-Escalation Tolerability Study
- 16 healthy male volunteers
- Single doses up to 800 mgwere well tolerated
Multiple Doses Tolerability
Study
- 16 healthy male volunteers
- 250, 600 mg doses daily for
7 days
Randomized Double-Blind, Placebo-Controlled
- 42 healthy individuals
- Olanzapine + placebo and olanzapine + BGP 15 400 mg daily for 17 days
Randomized Double-Blind, Placebo-Controlled Study
- 42 insulin resistant patients
- 200 or 400 mg BGP-15 or placebo daily for 28 days
Randomized, Double-blind, Placebo controlled
- 196 diabetic patients
- 100, 200 or 400 mg BGP-15 or placebo daily for 13 weeks
Serious Adverse Events
No Serious Adverse Events were recorded during the phase 2B study.
Treatment-Emergent Adverse Events
Neither TEAEs, nor blood chemistries, nor haematology, nor urinalysis did reveal any safety concerns for BGP-15
Most common TEAEs
Infections (33%), gastrointestinal (13%, e.g. bloating, constipation, diarrhea), nervous system related (11%, e.g. headache).
Compared to placebo
TEAEs were similar in severity, frequency and relatedness across placebo and active treatment groups, and there was no evident pattern across body system.
good safety profile
BGP-15 demonstrated
exceptionally good safety profile in all human clinical trials
Contact us
Get in touch
Want to know more about our research?
Let’s get in touch!
- info@vitaleonpharma.com
